Fda Eye Drop Recall 2025
Fda Eye Drop Recall 2025. Dozens of products are associated with a risk of eye infections that could result in partial vision loss or blindness. October 31, 2025 · 5 min read.
By aria bendix and rob wile. Last october, the agency warned 26 eye drop products could lead to a serious eye infection.
[1/31/2025] fda is warning consumers not to purchase or use south moon, rebright or fivfivgo eye drops because of the potential risk of eye infection.

Eye Drops Recall 2025 Full List of Brands, Retailers and More, [1/31/2025] fda is warning consumers not to purchase or use south moon, rebright or fivfivgo eye drops because of the potential risk of eye infection. A full list of potentially contaminated artificial tears.

FDA expands recall of eye drop products after reports of eye infections, In 2025, multiple eye drops were recalled. Dozens of products are associated with a risk of eye infections that could result in partial vision loss or blindness.
+1101902+eye+drops-Recall.jpg)
FDA Eye Drop Recall The Eye Associates, The number of recalled products reached a five. Which eye drops are being recalled?

FDA expands recall of eye drops linked to bacterial contamination Top, Last october, the agency warned 26 eye drop products could lead to a serious eye infection. Today, the fda issued a.

FDA Announces Recall on Several Eye Drop Brands Know Your Rights, An october recall of two dozen eyedrop brands came after fda staff found cracked floors, barefoot workers and other unsanitary conditions at a mumbai plant that. Global pharma healthcare is issuing a recall of its artificial tears lubricant eye drops that were distributed by ezricare and delsam pharma due to possible.
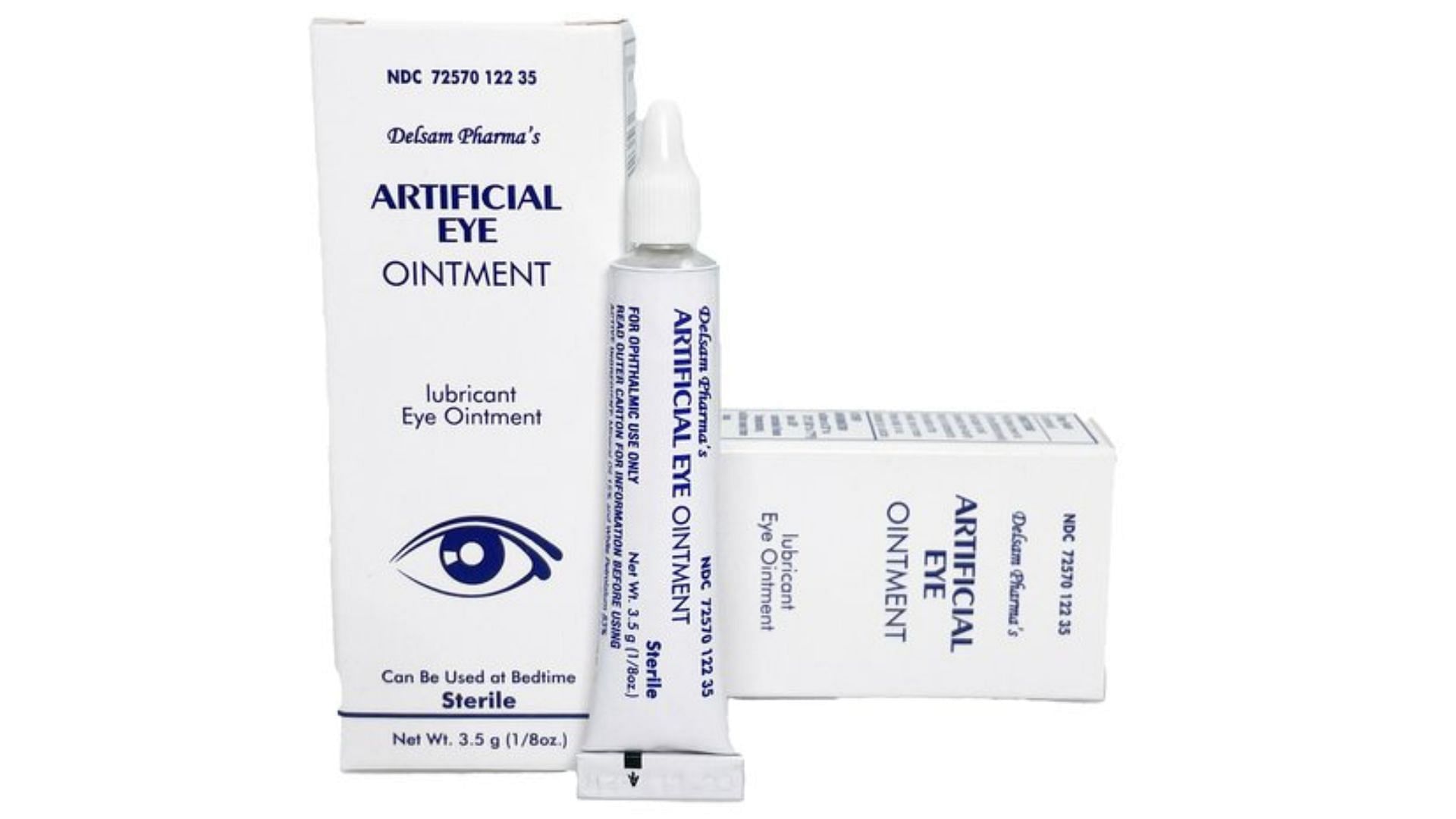
What eye drops are contaminated? FDA expands warning recall list, This recall is the latest in the fda’s increased focus on eye products. Four eye ointments sold at retailers such as cvs and walmart have been recalled after an fda investigation found that their production facility.
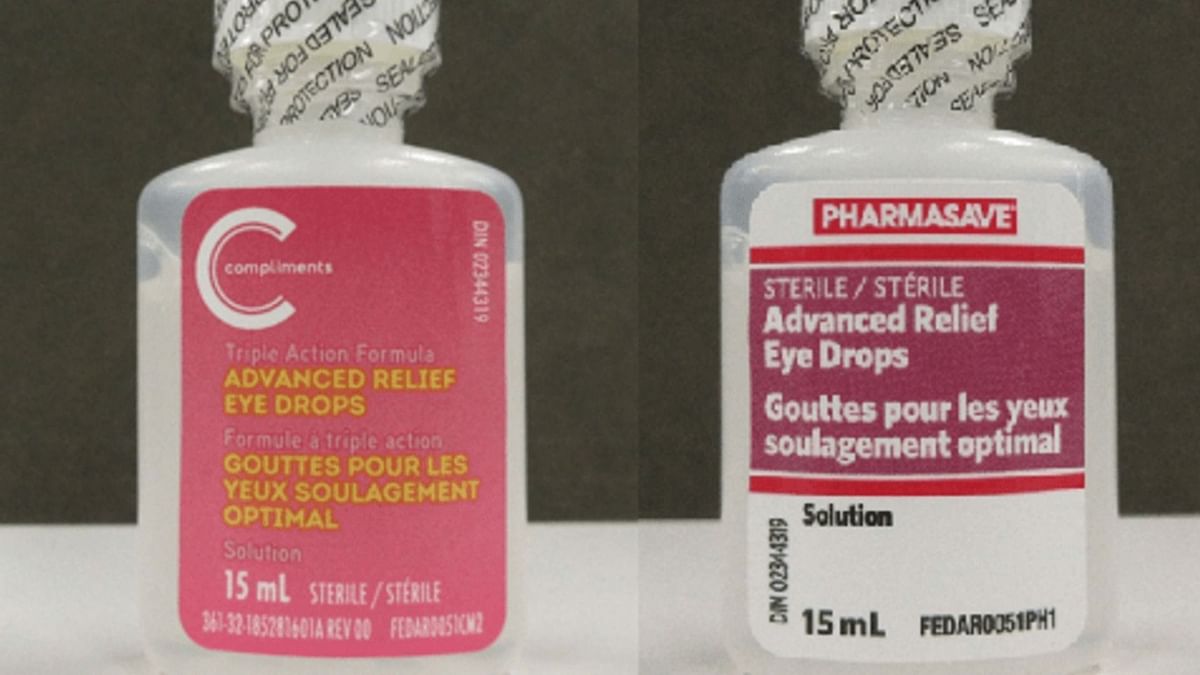
Eye Drops Recall 2025 Everything to know about Pharmasave and, By dennis thompson healthday reporter. March 8, 2025, 4:00 am pst.

FDA Eye Drops Recall Lawyer Contaminated Artificial Tears Attorney, Global pharma healthcare is issuing a recall of its artificial tears lubricant eye drops that were distributed by ezricare and delsam pharma due to possible. An october recall of two dozen eyedrop brands came after fda staff found cracked floors, barefoot workers and other unsanitary conditions at a mumbai plant that.
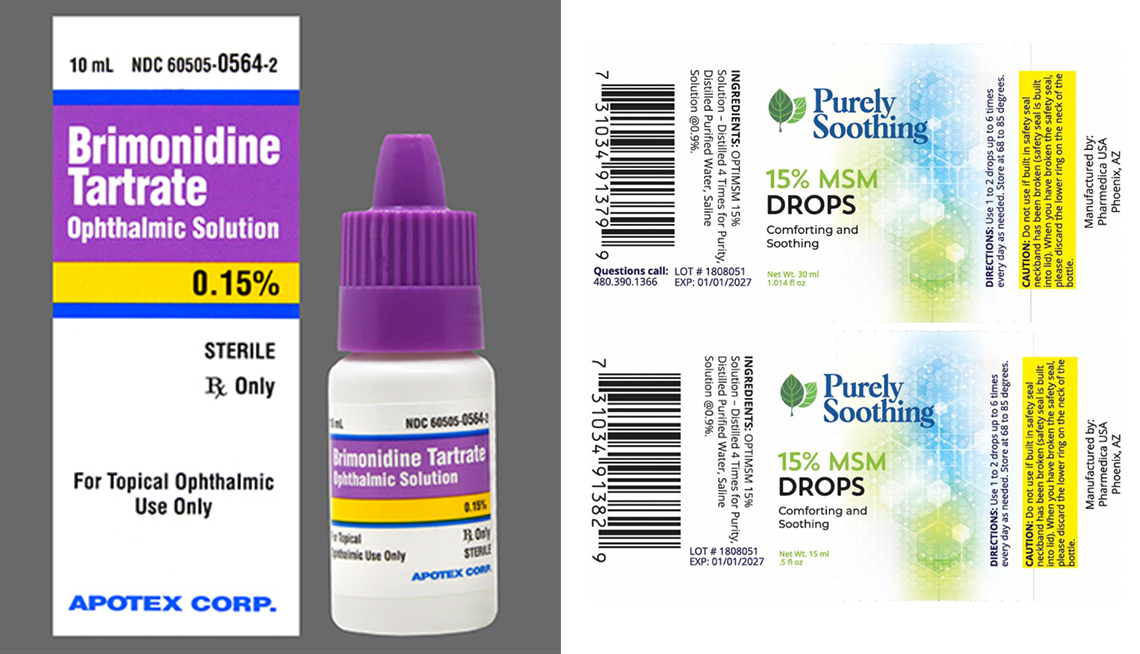
Eye drop recall, Global pharma healthcare is issuing a recall of its artificial tears lubricant eye drops that were distributed by ezricare and delsam pharma due to possible. [1/31/2025] fda is warning consumers not to purchase or use south moon, rebright or fivfivgo eye drops because of the potential risk of eye infection.

FDA Recalls NDC 49348037 Sunmark Eye Drops Original Formula Liquid, By dennis thompson healthday reporter. Global pharma healthcare is issuing a recall of its artificial tears lubricant eye drops that were distributed by ezricare and delsam pharma due to possible.
[1/31/2025] fda is warning consumers not to purchase or use south moon, rebright or fivfivgo eye drops because of the potential risk of eye infection.
Global pharma healthcare recalled artificial tears lubricant eye drops, distributed by ezricare and delsam pharma, due to possible bacterial contamination that.